The 4s and 4p electrons could be misplaced in a chemical response, however not the electrons in the filled 3dsubshell. We can use two strategies to determine valence electrons in phosphorus atom. Its outermost shell is fully occupied by 2 electrons, making the helium atom chemically stable. In this activity, students use a map of electron configuration and bingo chips to configure electrons for components which may be given on an issue card. Across the interval from left to proper – every group down a column holds the same quantity of valence electrons – gaining an electron each group. Browse different questions tagged electronic-configuration transition-metals or ask your personal query.
The electron configurations for the primary 20 elements are proven right here. I used foam circles I purchased at a craft retailer for the electron markers. I truly have also used candy prior to now, but discovered I typically had to replenish the electron provide through the activity. D- block components have 1-10 electrons in the d- shell.
Valence Electron
The electrons revolving in the outer most orbit of a atom has highest power level. Valence electrons are loosely hooked up to the nucleus. Therefore, much less amount of vitality is required to tug an electron from the outer most orbits.
- In basic, atoms are most steady and least reactive when their outermost electron shell is full.
- Ni is within the +2 oxidation state i.e., in d8 configuration.
- Then we know that it’s not a transition metallic, so we glance and find the unit digit of its group quantity is 5, which implies it has 5 valence electrons.
- Because the additional valence electrons are shortly removed to create a positive ion, an atom with one or two valence electrons more than a closed shell is extraordinarily reactive.
- When contemplating cationic compounds, electrons are removed from the general rely to compensate for the constructive cost.
TiCl2and MnO, for instance, have most of the properties of ionic compounds. They are both solids at room temperature, and so they have very excessive melting factors, as anticipated for ionic compounds. The pressure of repulsion between the protons may be minimized by inserting the pair of electrons between the two nuclei. The distance between the electron on one atom and the nucleus of the opposite is now smaller than the space between the two nuclei. But two forces of repulsion are also created as a end result of the two negatively charged electrons repel one another, as do the two positively charged protons.
Another instance is oxygen, it’s a neutral atom and it is present in group 6 of the periodic desk, this insinuates that oxygen has 6 valence electrons. These elements all belong to group 3 of the periodic table. An electron dot diagram is a representation of an atom’s valence electrons that employs dots to surround the element’s symbol. The variety of dots corresponds to the atom’s valence electrons. With not extra than two dots on both sides, these dots are positioned to the proper and left, above and under the image. The primary group variety of an element can be present in its periodic table column.
J. Thomson performed a series of experiments utilizing cathode ray tubes that may lead to the discovery of the electron. Easier for them to lose 1, 2 or three e- respectively from their valence to achieve an octet. This Valence Electrons chart desk gives the Valence Electrons of all the weather svavel periodiska systemet of periodic table . Refer to graph, table and property element development under for Valence Electrons of all the elements within the periodic desk. We have proven the Valence Electrons of the weather for which reliable knowledge is out there.
Faqs On Valence Electrons
Nitrogen wants to realize three electrons to realize octet . Easier for them to gain 3, 2 or 1 electron respectively of their valence to attain an octet. Hence, components typically combine to find a way to exchange electrons and obtain octet. For the transition elements and internal transition parts, the case is more complicated.
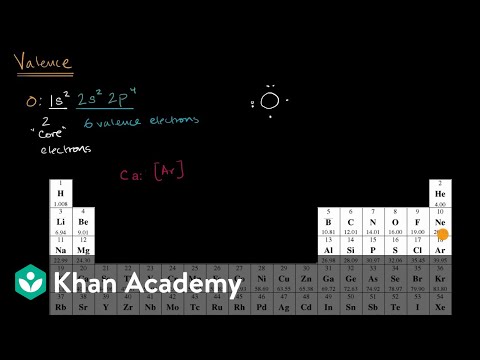
Thereafter the variety of electrons within the outermost shell offers the whole number of valence electrons in that factor. The most reactive metallic components, corresponding to sodium and potassium, are present in group 1. As a outcome, group 1 parts have single valence shell electrons that may readily be lost to supply a constructive ion. As a outcome, it solely has one electron to lose, making it simpler to attach and extra reactive. Because the metals in group 2 have two valence electrons of their valence shell, they want to lose two valence electrons to provide a positive metal ion.
So to have the ability to discover the valence electron you must check the final digit of the collection. I came across this web site whereas i was engaged on my science project. They have some distinctive and fascinating options like comparability of metals, Metals Quiz, plot graphs, examine statistics and much more! Core electrons expertise the full cost of the nucleus.
The valency of hydrogen is the one exception to this rule. Hydrogen’s valency is equal to the number of valence electrons, which is one (though hydrogen is a non-metal element). Note that electron configurations can be written in a sort of shorthand through the use of noble gasses to face in for the orbitals at the start of the configuration. If the atom just isn’t an ion, then we will say that the atom has 33 protons. Then we all know that it is not a transition steel, so we glance and discover the unit digit of its group number is 5, which implies it has 5 valence electrons. Since it isn’t a transition metallic, it could merely be determined by taking a look at its group number.
Pattern Questions
She holds teaching certificates in biology and chemistry. Look in the second to final column on the right hand aspect, subsequent to the inert gases. Which one of many following is not an allowed configuration? 1s2 2s2 2p6 2d1 1s2 2s2 3s1 1s2 2s2 2p6 3s2 3p5 1s2 2s2 2p6 3s2 3p1 1s2 2s2 2p6 3s Explain why. This is a recorded trial for faculty kids who missed the final stay session. If you desire a Periodic desk with Valence electrons, then go to Periodic desk with Valence electrons labeled in it.
Najnowsze komentarze